Reactivity of antipyrine and haloantipyrines in Pd-catalyzed C–H bond arylations
Résumé
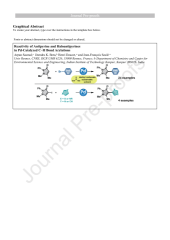
We reported herein the Pd-catalyzed direct arylation of antipyrine using Pd(OAc)2 as catalyst associated with KOAc as inexpensive base. In most cases, diethyl carbonate was used a sustainable solvent. The reaction tolerated a wide range of functional groups on the aryl bromide partners (e.g., nitrile, nitro, chloro, fluoro, formyl, acetyl, propionyl, benzoyl, ester, methyl, methoxy). In addition, some nitrogen-containing heteroaryl bromides were also efficiently coupled with antipyrine. We also demonstrated that in contrast to 4-bromoantipyrine, 4-iodoantipyrine could be employed as an efficient heteroaryl source in Pd-catalyzed C–H bond arylation of 5-membered ring heteroarenes.
Fichier principal
 Sasmal et al-2020-Reactivity of Antipyrine and Haloantipyrines in Pd-Catalyzed C–H Bond Arylations.pdf (612.94 Ko)
Télécharger le fichier
Sasmal et al-2020-Reactivity of Antipyrine and Haloantipyrines in Pd-Catalyzed C–H Bond Arylations.pdf (612.94 Ko)
Télécharger le fichier
Origine : Fichiers produits par l'(les) auteur(s)
Loading...

